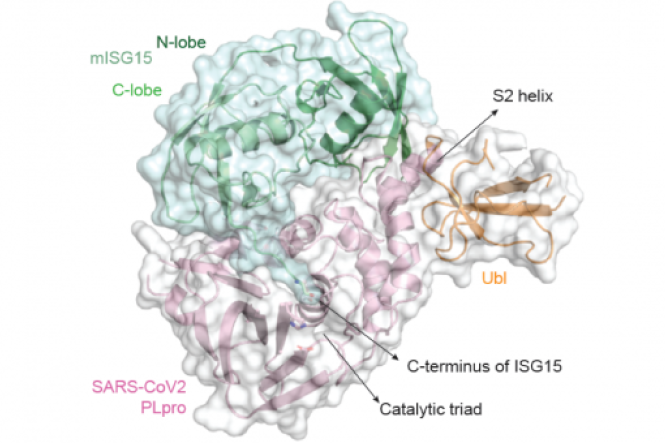
PLpro is required for the processing of viral polypeptides and the assembly of new viral particles within human cells. In addition, SARS-CoV-2 uses this enzyme to dampen the anti-viral immune response, helping the virus to modulate the host’s immune system to its own benefit. By this, the virus can easily multiply and spread further. The team has demonstrated that pharmaceutical targeting of PLpro by a non-covalent inhibitor (GRL-0617) blocks virus spread and increases anti-viral immunity in human epithelial cells, the prime site of pathogen entry. The respective results are now made publicly available and published in Nature.
“Our results offer the exciting possibility of a double-hit therapeutic strategy by targeting PLpro: stopping virus spread and empowering the human immune system at the same time”, explains IBC2 director Ivan Đikić, senior author on the manuscript. “To achieve this deep insight, we have combined biochemical, structural and functional expertise at our Institute, and I am incredibly thankful to the core team who has achieved a lot in a very short time. Our research alliance with the Institute of Medical Virology under the lead of Sandra Ciesek was of course the most critical to show how inhibition of PLpro stops the virus.“
Dr. Gerbrand van der Heden van Noort and Dr. Paul Geurink in the lab of late professor Huib Ovaa in the department of Cell and Chemical Biology of Leiden University Medical Centre chemically prepared tailor made assay reagents helping to further establish the PLpro preferences. Besides this, other scientists in Frankfurt, Munich, Mainz and Freiburg have contributed with experimental data, reagents, and advice.
These results deliver the full scientific rationale for pursuing further clinical investigations with compounds or drugs targeting PLpro of SARS-CoV-2. A US Clinic is currently performing clinical testing of the drug famotidine, proposed to target PLpro, in COVID-19 patients (Science magazine). “This is very exciting news. However, the on target activity of famotidine on viral PLpro has not yet been confirmed. Secondly, we need to be particularly careful in the clinic as the patients’ responses to novel anti-PLpro therapeutics may differ if applied in early or late stages of COVID-19” stress Đikić and Ciesek together.