Proteins are responsible for almost all cellular processes, from cell growth, to metabolism, the nervous system, and immune responses. Therefore, to understand how these biological processes work it is essential to study the proteins that drive these processes. Likewise, to understand how mutations alter the behaviour of proteins and cause diseases such as cancer, neurological diseases or immune disorders, it is essential to study the affected proteins in both their native and mutated form. Finally, the study of proteins also reveal how medicines bind to their target protein, how mutations in the target protein cause resistance, and how new medicines can be designed that are not susceptible to resistance.
To study the molecular mechanism of proteins, it necessary to study these in a purified form. However, obtaining pure protein is difficult as this requires specialized equipment and expertise. Therefore, the department of Cellular and Chemical Biology has set up a protein facility to produce, purify, and characterize protein, which will be open to all scientists at the LUMC. The facility has both bacterial and eukaryotic protein expression capabilities. High-throughput protein purification is provided by three Fast Protein Liquid Chromatography systems. The purity and stability of proteins will be analysed by thermal denaturation, size exclusion chromatography, multi-angle light scattering and dynamic light scattering. Interactions between proteins or protein and ligand will be analysed by isothermal titration calorimetry, bio-layer interferometry, or fluorescence-based approached such as FRET or fluorescence anisotropy. In addition, the purified proteins can be used in the high-throughput inhibitor screening platform (Ovaa lab) to find novel hit compounds for further drug development. Finally, the purified proteins can also be used for structural analysis by cryo-EM or protein X-ray crystallography. Access to cryo-EM is provided by the LUMC electron microscopy facility or the Netherlands Center for Electron Nanoscopy (NeCEN) located at the Institute for Biology Leiden. A protein crystallization facility is accessible at the Leiden Institute for Chemistry. Hence, the new Protein Facility will be a crucial new asset to the LUMC that will make it possible to study proteins in detail and find novel ways to combat disease.
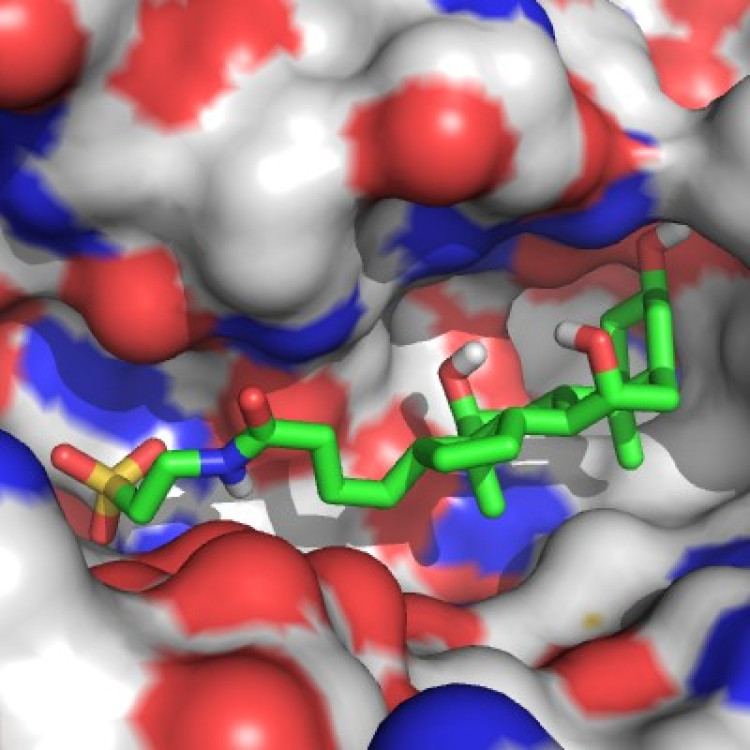
Close-up view of the exonuclease active site of the DnaE1 polymerase from the major pathogen Mycobacterium tuberculosis. A potential new inhibitor is shown in green sticks. The structure of the DnaE1 protein was determined by X-ray crystallography using purified protein. The inhibitor was selected through computational docking.



